Ru-catalysed C–H silylation of unprotected gramines, tryptamines and their congeners†
Abstract
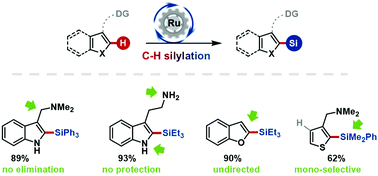
Selective Ru-catalysed C2–H silylation of heteroarenes is presented. The transformation works with or without directing group assistance and requires no protecting groups. Gramines and tryptamines may be converted efficiently whilst avoiding deleterious elimination side-reactions. Mechanistic studies reveal an unusual activation of the indole C4–H bond by an electron-rich metal.

 Please wait while we load your content...
Please wait while we load your content...