Full chirality transfer in the synthesis of hindered tertiary boronic esters under in situ lithiation–borylation conditions†
Abstract
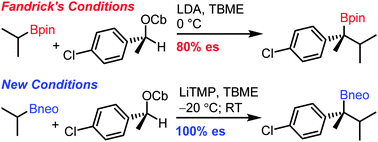
Hindered tertiary neopentyl glycol boronic esters can be prepared by using in situ lithiation–borylation of enantiopure secondary benzylic carbamates at −20 °C with full chirality transfer.

 Please wait while we load your content...
Please wait while we load your content...