Re-engineering a NiFe hydrogenase to increase the H2 production bias while maintaining native levels of O2 tolerance†
Abstract
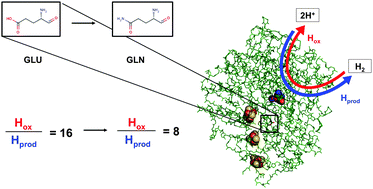
Naturally occurring oxygen tolerant NiFe membrane bound hydrogenases have a conserved catalytic bias towards hydrogen oxidation which limits their technological value. We present an Escherichia coli Hyd-1 amino acid exchange that apparently causes the catalytic rate of H2 production to double but does not impact the O2 tolerance.
- This article is part of the themed collection: 2016 Emerging Investigators

 Please wait while we load your content...
Please wait while we load your content...