Asymmetric sequential Au(i)/chiral tertiary amine catalysis: an enone-formation/cyanosilylation sequence to synthesize optically active 3-alkenyloxindoles from diazooxindoles†
Abstract
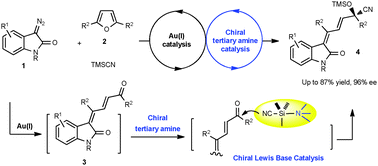
An unprecedented sequential gold-catalyzed enone-formation and bifunctional tertiary amine mediated asymmetric cyanosilylation reaction is developed, allowing the highly enantioselective synthesis of 3-alkenyloxindoles from diazooxindoles, furans and trimethylsilyl cyanide (TMSCN).

 Please wait while we load your content...
Please wait while we load your content...