Enantioselective bromocyclization of 2-geranylphenols induced by chiral phosphite–urea bifunctional catalysts†
Abstract
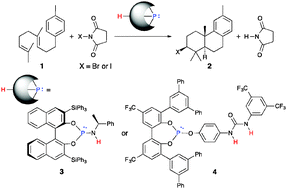
Chiral phosphite–urea bifunctional catalysts have been developed for the enantioselective bromocyclization of 2-geranylphenols with N-bromophthalimide (NBP) for the first time. The chiral triaryl phosphite moiety activates NBP to generate a bromophosphonium ion. On the other hand, the urea moiety interacts with a hydroxyl group of the substrate through hydrogen bonding interactions. Enantioselectivity is effectively induced through two-point attractive interactions between the catalyst and the substrate.

 Please wait while we load your content...
Please wait while we load your content...