Visible-light-initiated difluoromethylation of arene diazonium tetrafluoroborates†
Abstract
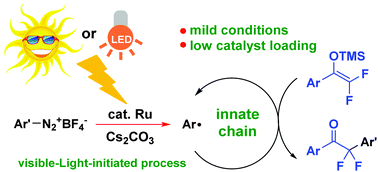
A mild and efficient method for the radical addition of α-aryl-β,β-difluoroenol silyl with arene diazonium tetrafluoroborates at room temperature has been disclosed, which involves an innate radical long chain cycle, so only a small amount (0.05 mol%) of photocatalyst and a short light exposure time are required as radical initiators. A proposed mechanism for the transformation is also illustrated based on the results of control experiments and quantum calculations. A variety of α-aryl-α,α-difluoroketones were formed in moderate to high yields, and can be easily further transformed into various difluoromethylarenes under basic conditions.

 Please wait while we load your content...
Please wait while we load your content...