Insights into metal–ligand hydrogen transfer: a square-planar ruthenate complex supported by a tetradentate amino–amido-diolefin ligand†
Abstract
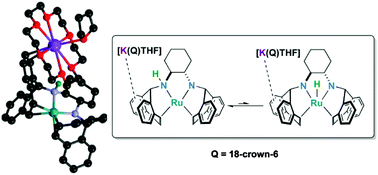
A four-coordinate, sixteen-electron Ru(0) complex containing the tetradentate diamino-diolefin ligand (±)-trans-N,N-bis(5H-dibenzo[a,d]cyclohepten-5-yl)-1,2-diaminocyclohexane (trop2dach) has been synthesised. Deprotonation of one amino N–H functional group generates an unprecedented four-coordinate ruthenate species which has been characterised in solution and in the solid state. The newly formed ruthenate complex undergoes intramolecular metal–ligand N–H addition/elimination in solution to generate a transient diamido ruthenium hydride species, as supported by NMR spectroscopy and density functional theory.
- This article is part of the themed collection: Celebrating the 2017 RSC Prize and Award Winners

 Please wait while we load your content...
Please wait while we load your content...