Metal free carboamination of internal alkynes – an easy access to polysubstituted quinolines†
Abstract
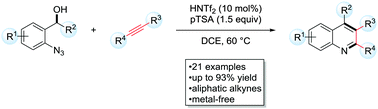
A metal free carboamination of unactivated alkynes towards highly substituted quinolines was realized in the presence of a synergistic Brønsted acid catalyst system. Supported by mechanistic probes, the reaction proceeds via a highly reactive vinyl cation in a C–C bond formation – Schmidt reaction sequence. The irreversible extrusion of N2, as a powerful driving force, allows for a general conversion of poorly nucleophilic aliphatic alkynes.

 Please wait while we load your content...
Please wait while we load your content...