Synthesis of activated 3′-amino-3′-deoxy-2-thio-thymidine, a superior substrate for the nonenzymatic copying of nucleic acid templates†
Abstract
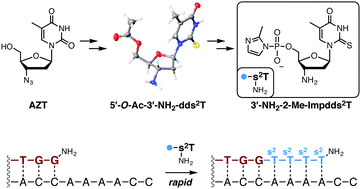
We present a scalable synthesis of 3′-amino-3′-deoxy-2-thio-thymidine-5′-phosphoro-2-methylimidazolide, an activated monomer that can copy adenosine residues in nucleic acid templates rapidly without a polymerase. The sulfur atom substitution enhances the rate of template copying by 5-fold compared with the 3′-amino-3′-deoxy-T monomer, while the 3′-amino monomers exhibit a 2- to 30-fold enhancement compared with their ribonucleotide counterparts.

 Please wait while we load your content...
Please wait while we load your content...