Radical C–H arylations of (hetero)arenes catalysed by gallic acid†
Abstract
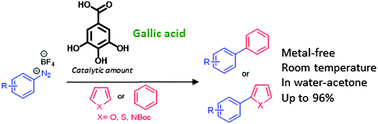
Gallic acid efficiently catalyses radical arylations in water–acetone at room temperature. This methodology proved to be versatile and scalable. Therefore, it constitutes a greener alternative to arylation. Moreover, considering that gallic acid is an abundant vegetable tannin, this work also unleashes an alternative method for the reutilisation of bio-wastes.
- This article is part of the themed collection: 2016 Emerging Investigators

 Please wait while we load your content...
Please wait while we load your content...