Dimerization of a marginally stable disilenyl germylene to tricyclic systems: evidence for reversible NHC-coordination†
Abstract
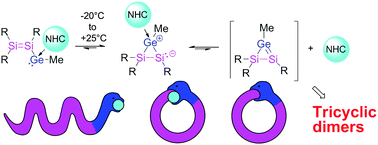
Tetrasiladigermatricyclohexanes in two isomeric forms (chair and doubly-bridged tetrahedron) are obtained by the reaction of MeLi with an α-chlorosilyl functionalized NHC-stabilized silagermenylidene. 29Si NMR at low temperature proves the initial formation of a monomeric NHC-adduct of a disilenyl germylene followed by cyclisation to the isomeric heavy cyclopropene. Addition of an excess of NHC stabilizes the both intermediates and demonstrates the reversibility of rate-determining initial equilibria involving NHC dissociation. Finally, a mixture of two isomeric tricyclic Si4Ge2 species is obtained: at elevated temperature the chair isomer converts to the doubly edge-bridged tetrahedron.

 Please wait while we load your content...
Please wait while we load your content...