3-Trifluoromethyl-3-aryldiazirine photolabels with enhanced ambient light stability†
Abstract
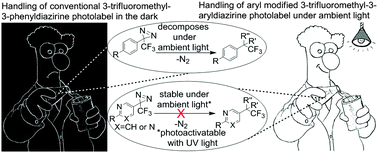
Ambient light stable 3-trifluoromethyl-3-aryldiazirine photolabels are developed via stabilization of the strained three membered diazirine ring by replacing the phenyl ring with electron withdrawing heterocyclic rings. Photolabeling studies reveal that these ambient light stable photolabels are equally efficient in photolabeling target proteins as the traditional 3-trifluoromethyl-3-phenyldiazirine and found to significantly increase the aqueous solubility of the photoaffinity labels.

 Please wait while we load your content...
Please wait while we load your content...