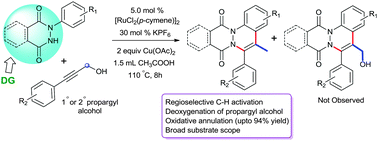
Expedient synthesis of new cinnoline diones by Ru-catalyzed regioselective unexpected deoxygenation-oxidative annulation of propargyl alcohols with phthalazinones and pyridazinones†
Abstract
Ruthenium-catalyzed simple, cascade and one-pot synthesis of cinnoline-fused diones has been carried out by the C–H activation of phthalazinones/pyridazinones accomplished by the unusual deoxygenation of propargyl alcohols. The bond selectivity is accredited to the traceless directing nature of the hydroxyl group of propargyl alcohol. A sequential C–H activation, insertion and deoxy-oxidative annulation has been proposed based on the preliminary mechanistic study.

 Please wait while we load your content...
Please wait while we load your content...