A mesoionic bis(Py-tzNHC) palladium(ii) complex catalyses “green” Sonogashira reaction through an unprecedented mechanism†‡
Abstract
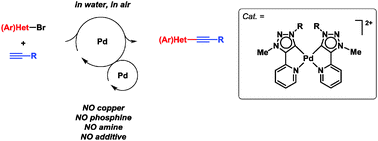
A novel bis(pyridyl-functionalized 1,2,3-triazol-5-ylidene)-palladium(II) complex [Pd(Py-tzNHC)2]2+ catalyses the copper-, amine-, phosphine-, and additive-free aerobic Sonogashira alkynylation of (hetero)aryl bromides in water as the only reaction solvent. The catalysis proceeds along two connected Pd-cycles with homogeneous bis-carbene Pd0 and PdII species, as demonstrated by electrospray ionization mass spectrometry.

 Please wait while we load your content...
Please wait while we load your content...