Mechanistic interrogation of the asymmetric lithiation-trapping of N-thiopivaloyl azetidine and pyrrolidine†
Abstract
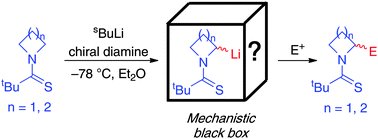
A fundamental mechanistic study of the s-BuLi/chiral diamine-mediated lithiation-trapping of N-thiopivaloyl azetidine and pyrrolidine is reported. We show that lithiated thiopivalamides are configurationally unstable at −78 °C. Reaction then proceeds via a dynamic resolution of diastereomeric lithiated intermediates and this accounts for the variable sense and degree of asymmetric induction observed compared to N-Boc heterocycles.


 Please wait while we load your content...
Please wait while we load your content...