One-pot sequential 1,2-addition, Pd-catalysed cross-coupling of organolithium reagents with Weinreb amides†
Abstract
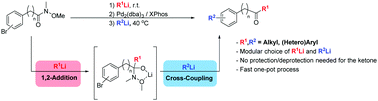
An efficient sequential 1,2-addition/cross-coupling of Weinreb amides with two organolithium reagents is reported. This synthetic approach allows access to a wide variety of functionalized ketones in a modular way. The one-pot procedure presented here takes advantage of a kinetically stable tetrahedral Weinreb intermediate during subsequent Pd-catalyzed cross-coupling with the second organolithium reagent leading, within short reaction times and under mild conditions, to the formation of ketones in excellent overall yields.

 Please wait while we load your content...
Please wait while we load your content...