Catalytic σ-activation of carbon–carbon triple bonds: reactions of propargylic alcohols and alkynes
Abstract
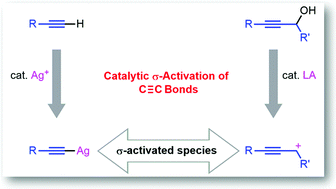
The majority reactions of alkynes in the literature are reported to proceed via either structural σ-activation or catalytic π-activation of C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C bonds. We skillfully designed novel methods for the catalytic σ-activation of C
C bonds. We skillfully designed novel methods for the catalytic σ-activation of C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C bonds of alkynyl compounds. For terminal alkynyl compounds, σ-activation was achieved by silver(I)-catalyzed C–H functionalization. Whereas σ-activation of internal alkynes was accomplished by the generation of propargylic cations from propargylic alcohols under Lewis-acid catalysis. These σ-activated species have been successfully used for new C–C and C–heteroatom bond formation reactions. Plausible reaction pathways were proposed based on typical control experiments to help the readers to gain insights into reactions and for further discovery of new reactions based on this concept of catalytic σ-activation of C
C bonds of alkynyl compounds. For terminal alkynyl compounds, σ-activation was achieved by silver(I)-catalyzed C–H functionalization. Whereas σ-activation of internal alkynes was accomplished by the generation of propargylic cations from propargylic alcohols under Lewis-acid catalysis. These σ-activated species have been successfully used for new C–C and C–heteroatom bond formation reactions. Plausible reaction pathways were proposed based on typical control experiments to help the readers to gain insights into reactions and for further discovery of new reactions based on this concept of catalytic σ-activation of C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C bonds.
C bonds.

 Please wait while we load your content...
Please wait while we load your content...