Tetra-cationic imidazoliumyl-substituted phosphorus–sulfur heterocycles from a cationic organophosphorus sulfide†
Abstract
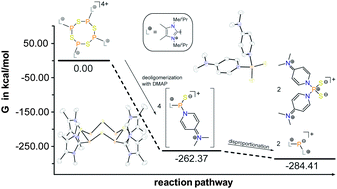
The reaction of imidazoliumyl-substituted P(III) cations of type [L(R,Me)PCl2]+ (3a,b+; LR,Me = imidazolium-2-yl a: R = Me; b: R = iPr) with (Me3Si)2S leads to the formation of tetra-cationic, eight-membered phosphorus sulfur heterocycles [L(R,Me)PS]44+ (9a,b4+), which can be explained by the tetramerization of the intermediately formed cationic phosphorus monosulfide [L(R,Me)PS]+ (8a,b+). The P4S4 ring adopts a crown conformation as observed for cyclo-S8. The Lewis base DMAP (4-dimethylaminopyridine) initiates a deoligomerization- and dismutation reaction of 9a,b4+ to give P(I) centered cation [L(R,Me)2P]+ (12a,b+) and phosphorus disulfide [(DMAP)2PS2]+ (14+).

 Please wait while we load your content...
Please wait while we load your content...