Design and synthesis of a new chromophore, 2-(4-nitrophenyl)benzofuran, for two-photon uncaging using near-IR light†
Abstract
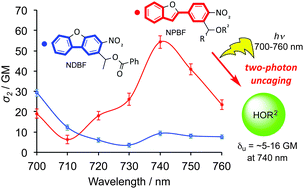
A new chromophore, 2-(4-nitrophenyl)benzofuran (NPBF), was designed for two-photon (TP) uncaging using near-IR light. The TP absorption (TPA) cross-sections of the newly designed NPBF chromophore were determined to be 18 GM at 720 nm and 54 GM at 740 nm in DMSO. The TP uncaging reaction of a caged benzoate with the NPBF chromophore quantitatively produced benzoic acid with an efficiency (δu) of ∼5.0 GM at 740 nm. The TP fragmentation of an EGTA unit was observed with δu = 16 GM. This behavior makes the new chromophore a promising TP photoremovable protecting group for physiological studies.


 Please wait while we load your content...
Please wait while we load your content...