Quantitative determination of betamethasone sodium phosphate and betamethasone dipropionate in human plasma by UPLC-MS/MS and a bioequivalence study
Abstract
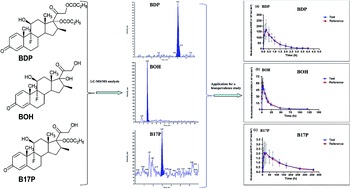
The compound medicine of betamethasone sodium phosphate (BSP) and betamethasone dipropionate (BDP) is widely used for the treatment of diverse glucocorticoid-sensitive acute and chronic diseases such as asthma, rheumatoid arthritis and systemic lupus erythematosus. It will be useful and beneficial to validate a sensitive method for the determination of BSP, BDP and their metabolites for a pharmacokinetic study. Hereby, ultra-high pressure liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) has been validated for the determination of BSP, BDP and their metabolites betamethasone (BOH), betamethasone 17-monodipropionate (B17P) and betamethasone 21-monodipropionate (B21P) in human plasma. Liquid–liquid extraction with ether and n-hexane (v/v, 4 : 1) was used for sample preparation of BDP, BOH, B17P and B21P with beclomethasone dipropionate as the internal standard (IS), while solid phase extraction was adopted for sample preparation of BSP using prednisolone as the IS. Chromatographic separation was performed on a Hypurity C18 column (150 mm × 2.1 mm, 5 μm) for BOH, BDP, B21P and B17P, and a Luna C18 (2) column (150 mm × 2.0 mm, 5 μm) for BSP. Electrospray ionization interfaced with positive multiple reaction monitoring (MRM) scan mode was used for mass spectrometric detection. The standard calibration curves were linear within the range of 2.525 × 10−9 to 403.9 × 10−9 mol dm−3 for BSP, 0.125 × 10−9 to 55.81 × 10−9 mol dm−3 for BDP, 0.278 × 10−9 to 74.95 × 10−9 mol dm−3 for BOH, 0.098 × 10−9 to 4.688 × 10−9 mol dm−3 for B17P and 0.226 × 10−9 to 5.411 × 10−9 mol dm−3 for B21P, respectively. The validated method was successfully applied to a bioequivalence study in 23 healthy subjects after they were injected with this compound medicine BSP and BDP.

 Please wait while we load your content...
Please wait while we load your content...