A validated stability-indicating HPLC-DAD method for simultaneous determination of econazole nitrate, triamcinolone acetonide, benzoic acid and butylated hydroxyanisole in cream dosage form
Abstract
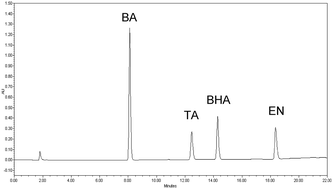
This study deals with the development and validation of a comprehensive stability-indicating high performance liquid chromatography with diode array detection (HPLC-DAD) method for simultaneous determination of econazole nitrate (EN), triamcinolone acetonide (TA), benzoic acid (BA) and butylated hydroxyanisole (BHA). To the best of our knowledge, no published methods could be found in the scientific literature for analysis of this quaternary mixture. Effective chromatographic separation was achieved using a Thermo Hypersil BDS C8 column (4.6 × 150 mm, 5 μm particle size) with gradient elution of the mobile phase composed of 0.2% w/v phosphoric acid (adjusted to pH 3.0 using ammonia solution) and methanol. The quantification of EN and BA was based on measuring their peak areas at 225 nm, while the quantification of TA and BHA was based on measuring their peak areas at 242 nm and 290 nm, respectively. BA, TA, BHA and EN peaks eluted at retention times of 8.11, 12.45, 14.28 and 18.36 min, respectively. Analytical performance of the proposed HPLC procedure was thoroughly validated with respect to system suitability, linearity ranges, precision, accuracy, specificity, robustness, and detection and quantification limits. The linearity ranges for EN, TA, BA and BHA were 1.5–300, 1–200, 0.6–100 and 1–100 μg mL−1, respectively, with correlation coefficients >0.9999. The analytes were subjected to forced-degradation conditions of neutral, acidic and alkaline hydrolysis, oxidation and thermal degradation. The proposed method proved to be stability-indicating by resolution of the analytes from their forced-degradation products. Moreover, specificity of the method was verified by resolution of the four analytes from more than 20 pharmaceutical compounds of various medicinal categories. The validated HPLC method was successfully applied to the analysis of the cited compounds in their cream dosage form. The proposed method made use of DAD as a tool for peak identity and purity confirmation.

 Please wait while we load your content...
Please wait while we load your content...