Fourier transform infrared spectroscopy on external perturbations inducing secondary structure changes of hemoglobin†
Abstract
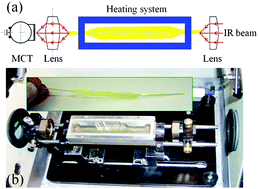
The secondary structure of proteins and their conformation are intimately related to their biological functions. In this study, heat-induced changes in the secondary structure and conformation of hemoglobin were investigated via infrared attenuated total reflection (IR-ATR) spectroscopy. The secondary structure changes of hemoglobin were derived from IR-ATR spectra using second derivatives and curve fitting. Thereby, the thermal denaturation temperature ranges and the secondary structure changes with temperature were revealed. More detailed information on the secondary structure and conformation was elucidated via two-dimensional infrared correlation spectroscopy. This study deciphers the detailed conformational behavior of hemoglobin molecular changes along with temperature, and creates a general methodological framework for analyzing the heat-induced behavior of biomacromolecules.

 Please wait while we load your content...
Please wait while we load your content...