Use of electrospinning and dynamic air focusing to create three-dimensional cell culture scaffolds in microfluidic devices†
Abstract
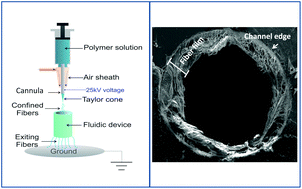
Organs-on-a-chip has emerged as a powerful tool for pharmacological and physiological studies. A key part in the construction of such a model is the ability to pattern or culture cells in a biomimetic fashion. Most of the reported cells-on-a-chip models integrate cells on a flat surface, which does not accurately represent the extracellular matrix that they experience in vivo. Electrospinning, a technique used to generate sub-micron diameter polymer fibers, has been used as an in vitro cell culture substrate and for tissue engineering applications. Electrospinning of fibers directly into a fully sealed fluidic channel using a conventional setup has not been possible due to issues of confining the fibers into a discrete network. In this work, a dynamic focusing method was developed, with this approach enabling direct deposition of electrospun fibers into a fully sealed fluidic channel, to act as a matrix for cell culture and subsequent studies under continuous flowing conditions. Scanning electron microscopy of electrospun polycaprolactone fibers shows that this method enables the formation of fibrous layers on the inner wall of a 3D-printed fluidic device (mean fiber size = 1.6 ± 0.6 μm and average pore size = 113 ± 19 μm2). Cells were able to be cultured in this 3D scaffold without the addition of adhesion proteins. Media was pumped through the channel at high flow rates (up to 400 μL min−1) during a dynamic cell culture process and both the fibers and the cells were found to be strongly adherent. A PDMS fluidic device was also prepared (from a 3D printed mold) and coated with polycaprolactone fibers. The PDMS device enables optical detection and confocal imaging of cultured cells on the fibers. Finally, macrophages were cultured in the devices to study how the fibrous scaffold can affect cell behavior. It was found that under lipopolysaccharide stimulation, macrophages cultured on PCL fibers inside of a channel secreted significantly more cytokines than those cultured on a thin layer of PCL in a channel or directly on the inner channel wall. Overall, this study represents a new approach for in vitro cell studies, where electrospinning can be used to easily and quickly create 3D scaffolds that can improve the culture conditions in microfluidic devices.
- This article is part of the themed collection: In memory of Craig Lunte

 Please wait while we load your content...
Please wait while we load your content...