An integrated, peptide-based approach to site-specific protein immobilization for detection of biomolecular interactions†
Abstract
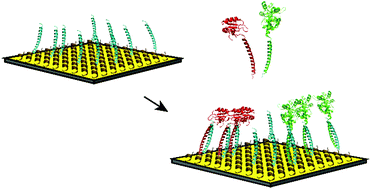
We have developed an integrated solution for the site-specific immobilization of proteins on a biosensor surface, which may be widely applicable for high throughput analytical purposes. The gold surface of a biosensor was coated with an anti-fouling layer of zwitterionic peptide molecules from which leucine zipper peptides protrude. Proteins of interest, the autoantigenic proteins La and U1A, were immobilized via a simple incubation procedure by using the complementary leucine zipper sequence as a genetically fused binding tag. This tag forms a strong coiled-coil interaction that is stable during multiple consecutive measurements and under common regeneration conditions. Visualization of the immobilized proteins of interest via antibody binding with multiplex surface plasmon resonance imaging demonstrated 2.5 times higher binding responses than when these proteins were randomly attached to the surface via the commonly applied activated ester-mediated coupling. The proteins could also be immobilized in a leucine zipper-dependent manner directly from complex mixtures like bacterial lysates, eliminating the need for laborious purification steps. This method allows the production of uniform functional protein arrays by control over immobilized protein orientation and geometry and is compatible with high-throughput procedures.


 Please wait while we load your content...
Please wait while we load your content...