Real-time monitoring of calcification process by Sporosarcina pasteurii biofilm†
Abstract
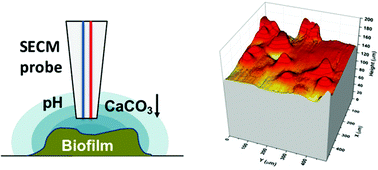
Sporosarcina pasteurii is known to produce calcite or biocement in the presence of urea and Ca2+. Herein, we report the use of novel ultramicrosensors such as pH, Ca2+, and redox sensors, along with a scanning electrochemical microscope (SECM), to monitor a real-time, bacteria-mediated urea hydrolysis process and subsequent changes in morphology due to CaCO3 precipitation. We report that the surface pH of a live biofilm changed rapidly from 7.4 to 9.2 within 2 min, whereas similar fast depletion (10 min) of Ca2+ was observed from 85 mM to 10 mM in the presence of a high urea (10 g L−1) brine solution at 23 °C. Both the pH and the Ca2+ concentration profiles were extended up to 600 μm from the biofilm surface, whereas the bulk chemical composition of the brine solution remained constant over the entire 4 h of SECM experiments. In addition, we observed a change in biofilm surface morphology and an increase in overall biofilm height of 50 μm after 4 h of precipitation. Electron microscopy confirmed the changes in surface morphology and formation of CaCO3 crystals. Development of the Ca2+ profile took 10 min, whereas that of the pH profile took 2 min. This finding indicates that the initial urea hydrolysis process is fast and limited by urease or number of bacteria, whereas later CaCO3 formation and growth of crystals is a slow chemical process. The ultramicrosensors and approaches employed here are capable of accurately characterizing bioremediation on temporal and spatial scales pertinent to the microbial communities and the processes they mediate.

 Please wait while we load your content...
Please wait while we load your content...