Design, fabrication and test of a pneumatically controlled, renewable, microfluidic bead trapping device for sequential injection analysis applications
Abstract
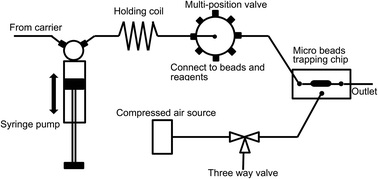
This paper describes the design, fabrication, and testing of a pneumatically controlled, renewable, microfluidic device for conducting bead-based assays in an automated sequential injection analysis system. The device used a “brick wall”-like pillar array (pillar size: 20 μm length × 50 μm width × 45 μm height) with 5 μm gaps between the pillars serving as the micro filter. The flow channel where bead trapping occurred is 500 μm wide × 75 μm deep. An elastomeric membrane and an air chamber were located underneath the flow channel. By applying pressure to the air chamber, the membrane is deformed and pushed upward against the filter structure. This effectively traps beads larger than 5 μm and creates a “bed” or micro column of beads that can be perfused and washed with liquid samples and reagents. Upon completion of the assay process, the pressure is released and the beads are flushed out from underneath the filter structure to renew the device. Mouse IgG was used as a model analyte to test the feasibility of using the proposed device for immunoassay applications. Resulting microbeads from an on-chip fluorescent immunoassay were individually examined using flow cytometry. The results show that the fluorescence signal intensity distribution is fairly narrow indicating high chemical reaction uniformity among the beads population. Electrochemical on-chip assay was also conducted. A detection limit of 1 ppb was achieved and good device reliability and repeatability were demonstrated. The novel microfluidic-based beads-trapping device thus opens up a new pathway to design micro-bead based immunoassays for various applications.

 Please wait while we load your content...
Please wait while we load your content...