Low-bandgap polymer electron acceptors based on double B ← N bridged bipyridine (BNBP) and diketopyrrolopyrrole (DPP) units for all-polymer solar cells†
Abstract
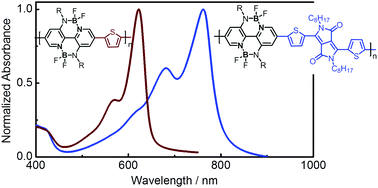
Broad absorption spectra and small optical bandgaps of polymer electron acceptors are very important for the sunlight harvesting of all-polymer solar cells (all-PSCs). Conjugated polymers based on the double B ← N bridged bipyridine (BNBP) unit are a new class of polymer electron acceptors, which suffer from narrow absorption spectra and large bandgaps. In this manuscript, we report a new polymer electron acceptor (P-BNBP-DPP) based on the BNBP unit and the dithienyl-diketopyrrolopyrrole (DPP) unit with a small bandgap and improved sunlight-harvesting capability. P-BNBP-DPP exhibits a broad absorption band with the onset absorbance at 796 nm and a small optical bandgap of 1.56 eV. Moreover, P-BNBP-DPP shows the low LUMO/HOMO energy levels of −3.87 eV/−5.45 eV and a high electron mobility of 2.1 × 10−4 cm2 V−1 s−1. An all-PSC device with P-BNBP-DPP as the acceptor and poly[(ethylhexyl-oxy)-benzodithiophene-(ethylhexyl)-thienothiophene] (PTB7) as the donor produces a power conversion efficiency of 2.69% with a broad external quantum efficiency response in the range of 300–800 nm. These results suggest an effective approach to tune the absorption spectra of BNBP-based polymer electron acceptors.

 Please wait while we load your content...
Please wait while we load your content...