Microparticles formulated from a family of novel silylated polysaccharides demonstrate inherent immunostimulatory properties and tunable hydrolytic degradability†
Abstract
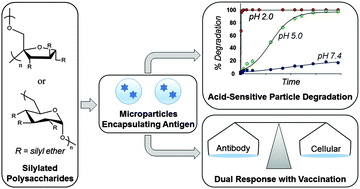
Acid-degradable polymers are well-suited for use as drug delivery vehicles because numerous physiological sites (e.g., intracellular endocytic pathway) are acidic. Here we report the synthesis of acid-sensitive silylated polysaccharides derived from either dextran or inulin with various alkyl substitutions on the silicon center: trimethylsilyl dextran (TMS-DEX), ethyldimethylsilyl dextran (EDMS-DEX), triethylsilyl dextran (TES-DEX), and trimethylsilyl inulin (TMS-IN). The silylated dextran (Silyl-DEX) and silylated inulin (Silyl-IN) polymers were fabricated into microparticles (MPs) via emulsification followed by solvent evaporation. These MPs were relatively stable at extracellular pH 7.4 and displayed a wide range of pH 2.0 and 5.0 degradation half-lives (fifteen minutes to greater than nine days) that were dependent on the extent of silylation (40 to 98%) and steric crowding on the silicon center (trimethyl to ethyldimethyl to triethyl). Silyl-DEX and Silyl-IN MPs exhibited cytocompatibility when cultured in vitro with RAW 264.7 macrophages. TES-DEX and TMS-IN MPs, composed of highly hydrophobic moieties and the parent immunostimulatory inulin, respectively, elicited substantial in vitro production of tumor necrosis factor alpha, a cytokine associated with an innate immune response. In vivo immunization with a model ovalbumin antigen encapsulated in silylated polysaccharide MPs, without a separate adjuvant, resulted in a dual humoral and cellular response that was superior to an alum-adjuvanted formulation. Overall, we present Silyl-DEX and Silyl-IN as members of the acid-degradable polymer family for potential use in subunit vaccines and other drug delivery applications.

 Please wait while we load your content...
Please wait while we load your content...