Impact of intermediate sites on bulk diffusion barriers: Mg intercalation in Mg2Mo3O8†
Abstract
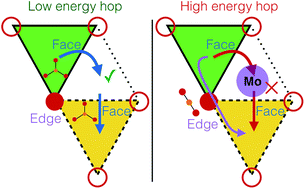
The ongoing search for high voltage positive electrode materials for Mg batteries has been primarily hampered by poor Mg mobility in bulk oxide frameworks. Motivated by the presence of Mo3 clusters that can facilitate charge redistribution and the presence of Mg in a non-preferred (tetrahedral) coordination environment, we have investigated the Mg (de)intercalation behavior in layered-Mg2Mo3O8, a potential positive electrode. While no electrochemical activity is observed, chemical demagnesiation of Mg2Mo3O8 is successful but leads to amorphization. Subsequent first-principles calculations predict a strong thermodynamic driving force for structure decomposition at low Mg concentrations and high activation barriers for bulk Mg diffusion, in agreement with experimental observations. Further analysis of the Mg diffusion pathway reveals an O–Mg–O dumbbell intermediate site that creates a high Mg2+ migration barrier, indicating the influence of transition states on setting the magnitude of migration barriers.
- This article is part of the themed collection: 2016 Journal of Materials Chemistry A HOT Papers


 Please wait while we load your content...
Please wait while we load your content...