MnSn2 negative electrodes for Na-ion batteries: a conversion-based reaction dissected
Abstract
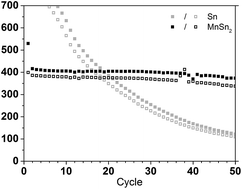
To date, the most common negative electrodes used in Na-ion batteries are based on hard carbons, offering around ca. 250 mAh g−1 gravimetric capacity but only 400 mAh cm−3 volumetric capacity due to their low density. Negative electrode materials based on intermetallics could outperform this with both a higher gravimetric capacity (>400 mAh g−1) and a higher volumetric capacity (>1000 mAh cm−3) but often struggle with cycling stability. Here MnSn2 is investigated as an electrode material for Na-ion batteries for the first time and delivers 400 mAh g−1 for over 50 cycles, by far outperforming its parent (Sn) in terms of cycling stability. The 1st cycle and the 10th cycle of the Na/MnSn2 reaction are probed using electrochemical methods and operando XRD to reveal the formation and ageing reaction mechanisms. It is shown that MnSn2 benefits from a robust reaction mechanism where all features seen in the 1st cycle (insertion into MnSn2, formation of Na15Sn4, Na15−xSn4, Na7Sn3 and MnSn2 reformation) are still visible in the 10th cycle, explaining the cycling stability.

 Please wait while we load your content...
Please wait while we load your content...