Catalyzed KSiH3 as a reversible hydrogen storage material†
Abstract
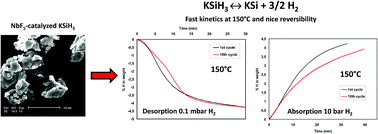
Solid-state hydrogen storage through the reversible formation of metallic hydrides is a key issue for the development of hydrogen as an energy vector. Herein we report the hydrogen storage performances of the KSiH3 phase ball-milled with NbF5 as a catalyst. The kinetics of hydrogen absorption/desorption are strongly enhanced by the addition of a catalyst as revealed by the large decrease of activation energies for both the absorption and desorption reactions. No disproportionation phenomenon is observed, indicating that the reaction between KSiH3 and KSi is perfectly reversible with a hydrogen storage capacity of 4.1 wt% H2. The thermodynamic properties of this KSi/KSiH3 equilibrium were investigated by plotting PCI curves from 90 °C to 130 °C: an enthalpy of 24.3 kJ mol−1 H2 and a low entropy change of 59.5 J K−1 mol−1 H2 are found. This low entropy variation is related to the high mobility of the H atoms in the α-KSiH3 phase as recently demonstrated by Quasi-Elastic Neutron Scattering (QENS) experiments.

 Please wait while we load your content...
Please wait while we load your content...