The goldilocks electrolyte: examining the performance of iron/nickel oxide thin films as catalysts for electrochemical water splitting in various aqueous NaOH solutions†
Abstract
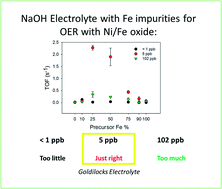
A rigorous study of electrodeposited pure and mixed Ni/Fe oxides was performed in three different sodium hydroxide electrolytes with various Fe impurity concentrations (<1 ppb, 5 ppb and 102 ppb). The presence and concentration of the Fe impurities in the three electrolytes is determined by Inductive Coupled Plasma spectroscopy. The rationale for investigating the OER performance of the pure and mixed Ni/Fe catalysts in various NaOH solutions, rather than the widely reported and more expensive KOH, with different Fe impurities was to conclude if the OER activity was comparable to the KOH and if the activity differed between NaOH solutions. A number of the mixed Ni/Fe catalysts in NaOH containing Fe impurities at a concentration of 5 ppb exhibited higher OER activities, with higher Turnover Frequency than the same catalyst in the NaOH solutions containing <1 ppb and 102 ppb and the state if the art RuO2. These Ni/Fe oxide materials are also cheaper to produce than the aforementioned platinum group materials therefore rendering these Ni/Fe catalysts more practical and economical. All of the material/electrolyte combinations are also evaluated with respect to their Tafel slopes and measured overpotential at a current density of 10 mA cm−2. To determine the Ni and Fe species formed before and after OER ex-situ Raman spectroscopy and X-ray photoelectron spectroscopy are utilised. Interestingly, the oxidation state of the Ni species in the pure Ni material does not change during OER in any of the NaOH media. While for the pure Fe and mixed Ni/Fe 50/50 material, the oxidation states of the species vary with the concentration of Fe impurities in the NaOH solution.

 Please wait while we load your content...
Please wait while we load your content...