Structural and electrochemical studies of a new Tavorite composition: LiVPO4OH†
Abstract
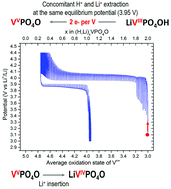
Polyanionic materials attract strong interest in the field of Li-ion battery research thanks to the wide range of compositions, structures and electrochemical properties they offer. Tavorite-type compositions offer a very rich crystal chemistry, among which LiVPO4F has the highest theoretical energy density (i.e. 655 W h kg−1). A new Tavorite-type LiVPO4OH composition was synthesized by a hydrothermal route from three different vanadium-containing precursors. The crystal structure of this new phase was fully determined thanks to synchrotron X-ray and neutron diffraction. 1H, 7Li, and 31P magic angle spinning nuclear magnetic resonance spectroscopy as well as diffuse reflectance infra-red spectroscopy were performed in order to support further the nature of the phases formed. Galvanostatic intermittent titration technique experiments in lithium batteries and ex situ X-ray diffraction analyses revealed that during oxidation the concomitant extraction of Li+ and H+ occurs at the same equilibrium potential (3.95 V vs. Li+/Li) and leads to the formation of the Tavorite phase VPO4O at the end of the charge. LiVPO4OH is also electrochemically active in the low voltage region, upon Li+ insertion. The reversible insertion/extraction of lithium at 1.35 V vs. Li+/Li leads to the formation of Li2VPO4OH at the end of discharge.

 Please wait while we load your content...
Please wait while we load your content...