Constructing efficient ion nanochannels in alkaline anion exchange membranes by the in situ assembly of a poly(ionic liquid) in metal–organic frameworks†
Abstract
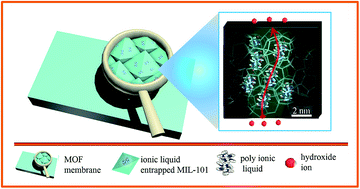
Alkaline anion exchange membranes (AEMs) have triggered great interest in the energy field because they permit the use of non-precious metal catalysts in fuel cells. It is of great significance to fabricate highly conductive AEMs by intensifying the ion transport within membranes through constructing ion nanochannels that provide efficient hydroxide ion transport. In this study, we propose a new approach to construct well-organized ion nanochannels by the in situ assembly of a poly(ionic liquid) (PIL) as the ion carrier within the highly ordered pores of metal–organic frameworks (MOFs). The MOF membrane, prepared by a facile hot-press method, exhibited a high conductivity of 36.6 mS cm−1 at a low ion concentration of 0.633 mmol cm−3 (20 °C), which is 6 orders of magnitude higher than that of the MIL-101 containing no poly(ionic liquid). Accordingly, the transport behavior of hydroxide ions was exploited, providing MOF membranes showing a high effective mobility of up to 6.597 × 10−4 cm2 s V−1 and a high transport hydroxide ion efficiency of up to 36.64% at 20 °C. The results imply that the efficiency of OH− conduction in the PIL confined MOF is 113% higher than that of the H+ conduction in Nafion.

 Please wait while we load your content...
Please wait while we load your content...