Rapid perovskite formation by CH3NH2 gas-induced intercalation and reaction of PbI2†
Abstract
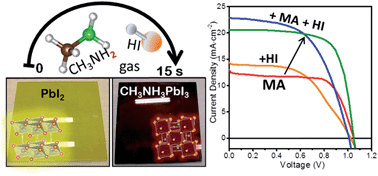
Solution processable perovskite solar cells traditionally use CH3NH3I solid powder as one of the two precursors that requires solvation into a solution and a spin-coating step; the resulting films need post-annealing (∼1 h) for complete conversion to CH3NH3PbI3. Here we describe for the first time the formation of stoichiometric perovskite in ambient air by exposing PbI2 films to a simple CH3NH2 gas precursor (as opposed to CH3NH3I solid powders). The reaction completes within a few seconds forming complete-coverage perovskite films with a roughness of 2 nm. The non-stoichiometric reaction produces Pb oxides as by-products, which are reconverted by further HI gas exposure. With combined measurements of the thin film crystal structure, chemical state, and absorption properties, we elucidate the chemical reaction mechanisms underlying these gas-induced processes. Fabricated solar cell devices show an efficiency of 15.3%, which remains almost the same after 133 days. Such a gas-induced reaction also enabled the successful preparation of high quality perovskite films with a size of 100 cm2.

 Please wait while we load your content...
Please wait while we load your content...