Predicting electrochemical properties and ionic diffusion in Na2+2xMn2−x(SO4)3: crafting a promising high voltage cathode material†
Abstract
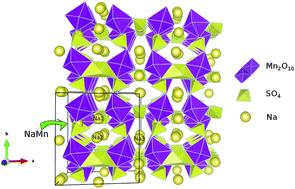
Sodium ion batteries have emerged as a good alternative to lithium based systems due to their low cost of production. In this scenario, the search for higher voltage, sodium cathodes results in a new promising alluaudite structure Na2+2xMn2−x(SO4)3. The structural, electronic and Na diffusion properties along with defects have been reported in this investigation within the framework of density functional theory. A band gap of 3.61 eV has been computed and the average deintercalation potential is determined to be 4.11 V vs. Na/Na+. A low concentration of anti-site defects is predicted due to their high formation energy. The biggest issue for the ionic diffusion in the Na2+2xMn2−x(SO4)3 crystal structure is revealed to be the effect of Mn vacancies increasing the activation energy of Na+ ions that hop along the [0 0 1] equilibrium positions. This effect leads to activation energies of almost the same high values for the ionic hop through the [0 1 0] direction characterizing a 2D like ionic diffusion mechanism in this system.
- This article is part of the themed collection: 2015 Journal of Materials Chemistry A Hot Papers

 Please wait while we load your content...
Please wait while we load your content...