Evaluation of Brønsted acidity and proton topology in Zr- and Hf-based metal–organic frameworks using potentiometric acid–base titration†
Abstract
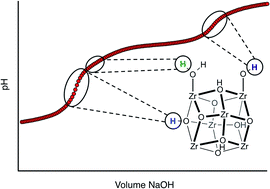
Potentiometric acid–base titration is introduced as a method to evaluate pKa values (Brønsted acidity) of protons present in the nodes of water stable Zr6- and Hf6-based metal–organic frameworks (MOFs), including UiO-type MOFs, NU-1000, and MOF-808. pKa values were determined for the three typical types of protons present in these MOFs: μ3-OH, M–OH2, and M–OH (M = Zr, Hf). Additionally, the data was used to quantify defect sites resulting from either a surfeit or shortage of linkers in the MOFs and to provide information about the true proton topology of each material.

 Please wait while we load your content...
Please wait while we load your content...