Benchmarking nanoparticulate metal oxide electrocatalysts for the alkaline water oxidation reaction†
Abstract
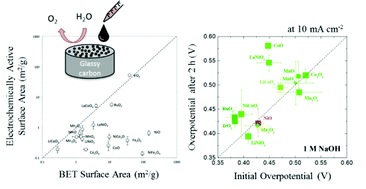
Nanoparticulate metal-oxide catalysts are among the most prevalent systems for alkaline water oxidation. However, comparisons of the electrochemical performance of these materials have been challenging due to the different methods of attachment, catalyst loadings, and electrochemical test conditions reported in the literature. Herein, we have leveraged a conventional drop-casting method that allows for the successful adhesion of a wide range of nanoparticulate catalysts to glassy-carbon electrode surfaces. We have applied this adhesion method to prepare catalyst films from 16 crystalline metal-oxide nanoparticles with a constant loading of 0.8 mg cm−2, and evaluated the resulting nanoparticulate films for the oxygen evolution reaction under conditions relevant to an integrated solar fuels device. In general, the activities of the adhered nanoparticulate films are similar to those of thin-film catalysts prepared by electrodeposition or sputtering, achieving 10 mA cm−2 current densities per geometric area at overpotentials of ∼0.35–0.5 V.
- This article is part of the themed collections: 2016 Journal of Materials Chemistry A Most Accessed Manuscripts and Water splitting and photocatalysis

 Please wait while we load your content...
Please wait while we load your content...