Highly efficient rutile TiO2 photocatalysts with single Cu(ii) and Fe(iii) surface catalytic sites†
Abstract
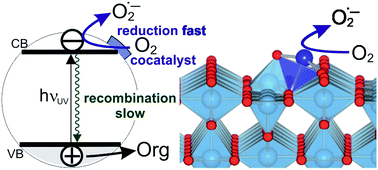
Highly active photocatalysts were obtained by impregnation of nanocrystalline rutile TiO2 powders with small amounts of Cu(II) and Fe(III) ions, resulting in the enhancement of initial rates of photocatalytic degradation of 4-chlorophenol in water by factors of 7 and 4, compared to pristine rutile, respectively. Detailed structural analysis by EPR and X-ray absorption spectroscopy (EXAFS) revealed that Cu(II) and Fe(III) are present as single species on the rutile surface. The mechanism of the photoactivity enhancement was elucidated by a combination of DFT calculations and detailed experimental mechanistic studies including photoluminescence measurements, photocatalytic experiments using scavengers, OH radical detection, and photopotential transient measurements. The results demonstrate that the single Cu(II) and Fe(III) ions act as effective cocatalytic sites, enhancing the charge separation, catalyzing “dark” redox reactions at the interface, thus improving the normally very low quantum yields of UV light-activated TiO2 photocatalysts. The exact mechanism of the photoactivity enhancement differs depending on the nature of the cocatalyst. Cu(II)-decorated samples exhibit fast transfer of photogenerated electrons to Cu(II/I) sites, followed by enhanced catalysis of dioxygen reduction, resulting in improved charge separation and higher photocatalytic degradation rates. At Fe(III)-modified rutile the rate of dioxygen reduction is not improved and the photocatalytic enhancement is attributed to higher production of highly oxidizing hydroxyl radicals produced by alternative oxygen reduction pathways opened by the presence of catalytic Fe(III/II) sites. Importantly, it was demonstrated that excessive heat treatment (at 450 °C) of photocatalysts leads to loss of activity due to migration of Cu(II) and Fe(III) ions from TiO2 surface to the bulk, accompanied by formation of oxygen vacancies. The demonstrated variety of mechanisms of photoactivity enhancement at single site catalyst-modified photocatalysts holds promise for developing further tailored photocatalysts for various applications.
- This article is part of the themed collection: Water splitting and photocatalysis

 Please wait while we load your content...
Please wait while we load your content...