Oxadendralenes in asymmetric organocatalysis for the construction of tetrahydroisochromenes†
Abstract
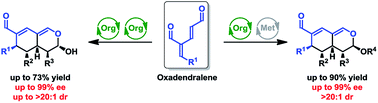
Oxadendralenes are integrated in a novel manner into a one-pot cascade utilizing synergistic catalysis for the construction of valuable and complex bicyclic heterocyclic scaffolds. The construction is based on the organocatalytic activation of the oxadendralenes generating a vinylogous iminium-ion intermediate which is set-up for a 1,6-addition with an enamine formed from an aldehyde and the same organocatalyst. This reaction generates a cyclic oxadendralenic intermediate, which acts as an electron-deficient heterodiene reacting in a Lewis-acid catalyzed hetero-Diels–Alder reaction with vinyl ethers to form tetrahydroisochromenes with five continuous stereocenters in high yields, >20 : 1 dr and 99% ee. This synergistic organo- and Lewis-acid catalysed system also displays high tolerance for variation in oxadendralenes and aldehydes, which provides tetrahydroisochromenes with high diversity in the substituent pattern and the same excellent stereoselectivities. Mechanistic studies have been performed to account for the activation modes and stereochemical outcome of the reaction. The reaction concept has been extended to also include a sequential organocatalytic reaction of oxadendralenes with aldehydes, in which the enamine formed from the aldehyde and the organocatalyst act both in the first catalytic cycle forming the cyclic oxadendralenic intermediate and in a second catalytic cycle leading to tetrahydroisochromenes in good yields and excellent stereoselectivities. Mechanistic studies reveal that the stereochemistry of the organocatalyst has an influence on the diastereoselectivity of the reaction sequence. Some transformations of the tetrahydroisochromenes are also presented. The chiral tetrahydroisochromenes formed might be applied in the diversified synthesis of important drugs.

 Please wait while we load your content...
Please wait while we load your content...