Abhijnan Ray Choudhury and Santanu Mukherjee
Chem. Sci., 2016,7, 6940-6945
DOI:
10.1039/C6SC02466A,
Edge Article
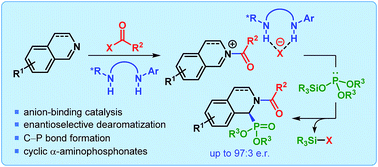
An enantioselective dearomatization of isoquinolines has been developed using chiral anion-binding catalysis. This transformation, catalyzed by a simple and easy to prepare tert-leucine-based thiourea derivative, makes use of silyl phosphite as a nucleophile and generates cyclic α-aminophosphonates. This is the first time asymmetric anion-binding catalysis has been applied to the synthesis of α-aminophosphonates.