Visible light mediated metal-free thiol–yne click reaction†
Abstract
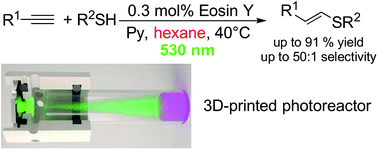
The carbon–sulfur bond formation reaction is of paramount importance for functionalized materials design, as well as for biochemical applications. The use of expensive metal-based catalysts and the consequent contamination with trace metal impurities are challenging drawbacks of the existing methodologies. Here, we describe the first environmentally friendly metal-free photoredox pathway to the thiol–yne click reaction. Using Eosin Y as a cheap and readily available catalyst, C–S coupling products were obtained in high yields (up to 91%) and excellent selectivity (up to 60 : 1). A 3D-printed photoreactor was developed to create arrays of parallel reactions with temperature stabilization to improve the performance of the catalytic system.

 Please wait while we load your content...
Please wait while we load your content...