Zn⋯Zn interactions at nickel and palladium centers†
Abstract
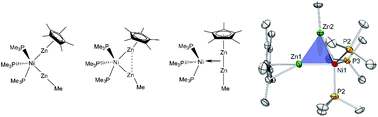
The analogy between ZnR fragments and the hydrogen radical represents a fruitful concept in organometallic synthesis. The organozinc(II) and -zinc(I) sources ZnMe2 (Me = methyl) and [Zn2Cp*2] (Cp* = pentamethylcyclopentadienyl) provide one-electron fragments ·ZnR (R = Me, Cp*), which can be trapped by transition metal complexes [LaM], yielding [Lb(ZnR)n]. The addition of the dizinc compound [Zn2Cp*2] to coordinatively unsaturated [LaM] by the homolytic cleavage of the Zn–Zn bond can be compared to the classic oxidative addition reaction of H2, forming dihydride complexes [LaM(H)2]. It has also been widely shown that dihydrogen coordinates under preservation of the H–H bond in the case of certain electronic properties of the transition metal fragment. The σ-aromatic triangular clusters [Zn3Cp*3]+ and [Zn2CuCp*3] may be regarded as the first indication of this so far unknown, side-on coordination mode of [Zn2Cp*2]. With this background in mind the question arises if a series of complexes featuring the Zn2M structural motif can be prepared exhibiting a (more or less) intact Zn–Zn interaction, i.e. di-zinc complexes which are analogous to non-classical dihydrogen complexes of the Kubas type. In order to probe this idea, a series of interrelated organozinc nickel and palladium complexes and clusters were synthesized and characterized as model compounds: [Ni(ZnCp*)(ZnMe)(PMe3)3] (1), [Ni(ZnCp*)2(ZnMe)2(PMe3)2] (2), [{Ni(CNtBu)2(μ2-ZnCp*)(μ2-ZnMe)}2] (3), [Pd(ZnCp*)4(CNtBu)2] (4) and [Pd3Zn6(PCy3)2(Cp*)4] (5). The dependence of Zn⋯Zn interactions as a function of the ligand environments and the metal centers was studied. Experimental X-ray crystallographic structural data and DFT calculations support the analogy between dihydrogen and dizinc transition metal complexes.

 Please wait while we load your content...
Please wait while we load your content...