Nucleophile promoted gold redox catalysis with diazonium salts: C–Br, C–S and C–P bond formation through catalytic Sandmeyer coupling†
Abstract
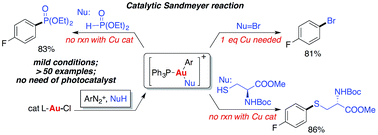
Gold-catalyzed C-heteroatom (C–X) coupling reactions are evaluated without using sacrificial oxidants. Vital to the success of this methodology is the nucleophile-assisted activation of aryldiazonium salts, which could be an effective oxidant for converting Au(I) to Au(III) even without the addition of an assisting ligand or photocatalyst. By accelerating the reaction kinetics to outcompete C–C homo-coupling or diazonium dediazoniation, gold-catalyzed Sandmeyer reactions were achieved with different nucleophiles, forming C–Br, C–S and C–P bonds in high yields and selectivities.

 Please wait while we load your content...
Please wait while we load your content...