Elucidation of the heme active site electronic structure affecting the unprecedented nitrite dismutase activity of the ferriheme b proteins, the nitrophorins†
Abstract
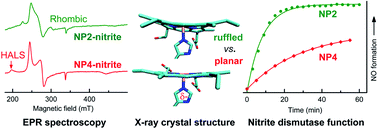
Nitrophorins (NPs) catalyze the nitrite dismutation reaction that is unprecedented in ferriheme proteins. Despite progress in studying the reaction mechanism, fundamental issues regarding the correlation of the structural features with the nitrite dismutase activity of NPs remain elusive. On the other hand, it has been shown that the nitrite complexes of NPs are unique among those of the ferriheme proteins since some of their electron paramagnetic resonance (EPR) spectra show significant highly anisotropic low spin (HALS) signals with large gmax values over 3.2. The origin of HALS signals in ferriheme proteins or models is not well understood, especially in cases where axial ligands other than histidine are present. In this study several mutations were introduced in NP4. The related nitrite coordination and dismutation reaction were investigated. As a result, the EPR spectra of the NP–nitrite complexes were found to be tightly correlated with the extent of heme ruffling and protonation state of the proximal His ligand—dictated by an extended H-bonding network at the heme active site. Furthermore, it is established that the two factors are essential in determining the nitrite dismutase activity of NPs. These results may provide a valuable guide for identifying or designing novel heme proteins with similar activity.

 Please wait while we load your content...
Please wait while we load your content...