Highly selective catalytic trans-hydroboration of alkynes mediated by borenium cations and B(C6F5)3†
Abstract
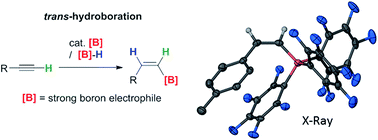
The trans-hydroboration of terminal alkynes mediated by borenium cations [NHC(9-BBN)]+ (NHC = N-heterocyclic carbene, 9-BBN = 9-borabicyclo(3.3.1)nonane) exclusively affords Z-vinylboranes. NHCs and chelating dialkyl substituents on the borenium cation and “non”-basic anions were essential to preclude alternative reactions including dehydroboration. Deuterium labelling studies indicate the mechanism involves addition of the boron electrophile to the alkyne and transfer of hydride to the opposite face of the activated alkyne. trans-Hydroboration proceeds with only catalytic amounts of B(C6F5)3 or [Ph3C][B(C6F5)4] to activate the (NHC)9-BBN(H) precursor with the borenium regenerated in the hydride transfer step. The NHC can be removed from the trans-hydroborated products by the addition of Et2O–BF3 providing access to vinylBBN species effective for Suzuki–Miyaura couplings to generate Z-alkenes. Combinations of catalytic B(C6F5)3 and stoichiometric [HB(C6F5)3]− also lead to trans-hydroboration of terminal alkynes to form Z-isomers of [arylCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHB(C6F5)3]−.
CHB(C6F5)3]−.


 Please wait while we load your content...
Please wait while we load your content...