Structural engineering of porphyrin-based small molecules as donors for efficient organic solar cells†
Abstract
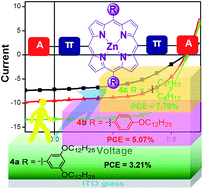
Porphyrin-based small molecules as donors have long been ignored in bulky heterojunction organic solar cells due to their unfavorable aggregation and the low charge mobility. With the aim of striking a delicate balance between molecular design, morphology, interfacial layer and device fabrication to maximize the power conversion efficiency (PCE) of organic solar cells, three comparable porphyrin-based small molecules with an acceptor–donor–acceptor configuration have been developed for use as donor materials in solution processed small molecule bulk heterojunction organic solar cells. In these molecules, electron-deficient 3-ethylrhodanine is introduced into the electron-rich porphyrin core through 5,15-bis(phenylethynyl) linkers. Structural engineering with 10,20-bis(2-hexylnonyl) aliphatic peripheral substituent on the porphyrin core, instead of the aromatic substituents such as 10,20-bis[3,5-di(dodecyloxyl)phenyl], and 10,20-bis(4-dodecyloxylphenyl), can simultaneously facilitate stronger intermolecular π–π stacking and higher charge transfer mobility in the film, leading to a maximum PCE of 7.70% in a conventional device. The inverted devices have also been demonstrated to have long-term ambient stability and a comparable PCE of 7.55%.
- This article is part of the themed collection: Global Energy Challenges: Solar Energy

 Please wait while we load your content...
Please wait while we load your content...