A single cation or anion dendrimer-based liquid electrolyte†
Abstract
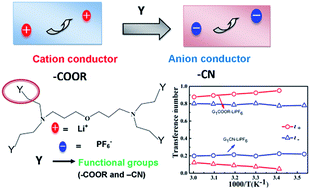
We propose here a novel liquid dendrimer-based single ion conductor as a potential alternative to conventional molecular liquid solvent–salt solutions in rechargeable batteries, sensors and actuators. A specific change from ester (–COOR) to cyano (–CN) terminated peripheral groups in generation-one poly(propyl ether imine) (G1-PETIM)–lithium salt complexes results in a remarkable switchover from a high cation (tLi+ = 0.9 for –COOR) to a high anion (tPF6− = 0.8 for –CN) transference number. This observed switchover draws an interesting analogy with the concept of heterogeneous doping, applied successfully to account for similar changes in ionic conductivity arising out of dispersion of insulator particle inclusions in weak inorganic solid electrolytes. The change in peripheral group simultaneously affects the effective ionic conductivity, with the room temperature ionic conductivity of PETIM–CN (1.9 × 10−5 Ω−1 cm−1) being an order of magnitude higher than PETIM–COOR (1.9 × 10−6 Ω−1 cm−1). Notably, no significant changes are observed in the lithium mobility even following changes in viscosity due to the change in the peripheral group. Changes in the peripheral chemical functionality directly influence the anion mobility, being lower in PETIM–COOR than in PETIM–CN, which ultimately becomes the sole parameter controlling the effective transport and electrochemical properties of the dendrimer electrolytes.
- This article is part of the themed collection: Global Energy Challenges: Energy Applications

 Please wait while we load your content...
Please wait while we load your content...