Quantitative model for rationalizing solvent effect in noncovalent CH–Aryl interactions†
Abstract
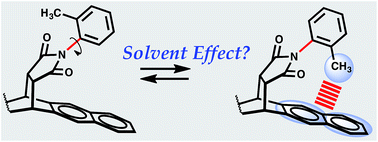
The strength of CH–aryl interactions (ΔG) in 14 solvents was determined via the conformational analysis of a molecular torsion balance. The molecular balance adopted folded and unfolded conformers in which the ratio of the conformers in solution provided a quantitative measure of ΔG as a function of solvation. While a single empirical solvent parameter based on solvent polarity failed to explain solvent effect in the molecular balance, it is shown that these ΔG values can be correlated through a multiparameter linear solvation energy relationship (LSER) using the equation introduced by Kamlet and Taft. The resulting LSER equation [ΔG = −0.24 + 0.23α − 0.68β − 0.1π* + 0.09δ]—expresses ΔG as a function of Kamlet–Taft solvent parameters—revealed that specific solvent effects (α and β) are mainly responsible for “tipping” the molecular balance in favour of one conformer over the other, where α represents a solvents' hydrogen-bond acidity and β represents a solvents' hydrogen-bond basicity. Furthermore, using extrapolated data (α and β) and the known π* value for the gas phase, the LSER equation predicted ΔG in the gas phase to be −0.31 kcal mol−1, which agrees with −0.35 kcal mol−1 estimated from DFT-D calculations.

 Please wait while we load your content...
Please wait while we load your content...