A thorough anion–π interaction study in biomolecules: on the importance of cooperativity effects†
Abstract
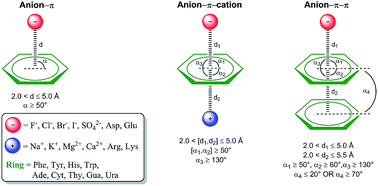
Noncovalent interactions have a constitutive role in the science of intermolecular relationships, particularly those involving aromatic rings such as π–π and cation–π. In recent years, anion–π contact has also been recognized as a noncovalent bonding interaction with important implications in chemical processes. Yet, its involvement in biological processes has been scarcely reported. Herein we present a large-scale PDB analysis of the occurrence of anion–π interactions in proteins and nucleic acids. In addition we have gone a step further by considering the existence of cooperativity effects through the inclusion of a second noncovalent interaction, i.e. π-stacking, T-shaped, or cation–π interactions to form anion–π–π and anion–π–cation triads. The statistical analysis of the thousands of identified interactions reveals striking selectivities and subtle cooperativity effects among the anions, π-systems, and cations in a biological context. The reported results stress the importance of anion–π interactions and the cooperativity that arises from ternary contacts in key biological processes, such as protein folding and function and nucleic acids–protein and protein–protein recognition. We include examples of anion–π interactions and triads putatively involved in enzymatic catalysis, epigenetic gene regulation, antigen–antibody recognition, and protein dimerization.


 Please wait while we load your content...
Please wait while we load your content...